
ClinCare EDC
ClinCare® EDC is a Software as a Service (SaaS) based integrated solution optimized for data capture and data entry. The platform facilitates the collection, management, and reporting of clinical study data through a secure, web-based interface.
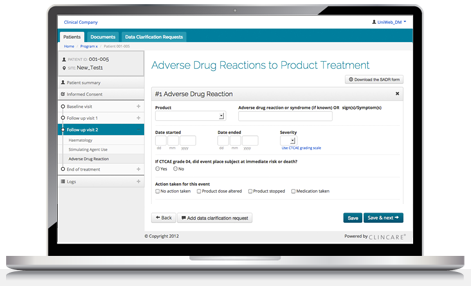
As an EDC tool, ClinCare EDC enables bio-pharma companies, independent clinics, and CROs to efficiently conduct clinical trials (phases I-IV and all therapeutic areas), medical needs programs, registries, and patient reported outcome.
A 3rd-generation system built by a team of experts, ClinCare EDC is a proven technology used in many studies. The system facilitates rapid study build and deployment. ClinCare EDC provides a streamlined system that coordinates clinical data collection across the research team and securely manages data during any phase of the the clinical trial process. Full query management, both automatic and manually generated, is integrated for rapid query resolution. Likewise, an audit trail with justification of changes has been incorporated into the system to track all study actions within the system. Created with an intuitive user interface, usability from the perspective of all users is a strength. Additionally, our team has the ability to design custom features to fit study-specific goals.
ClinCare EDC is designed for use by all members of the clinical research team: investigators/study nurses, clinical research associates, project managers, data managers, sponsors, and user administrators.
CROs and bio-pharma companies can have confidence that ClinCare EDC will facilitate better communication and efficient work practices through streamlined workflow on a secure platform.
ClinCare EDC will improve your research efficiency at an affordable cost while providing built in 21 CFR Part 11 and regulatory compliance.
Features
ClinCare EDC comes standard with a comprehensive set of features for securely capturing and managing clinical data.
Major features and functionalities:
- Data Capture: inclusion, visits, input validation
- Monitoring: source data verification
- Management of Data: review data, generate queries
- Extract Data: extracting clinical data sets
- Administration: auditing, configuration, and reporting
- Audit trail: with justification of changes
- Randomization module
- Double Data Entry module
Apart from the rich feature set, the strength of our solution lies in the thorough customization possibilities to match study needs. ClinCare has the distinct ability to adapt to customer processes, designing any new features necessary for customer-specific study needs in weeks.
Applications
Clinical Trials
ClinCare EDC provides a streamlined system that coordinates clinical data collection across your research team and securely manages your data during any phase (phases I, II, III, IV) of the clinical trial process while providing built-in 21 CFR Part 11 and regulatory compliance.
Registries
Registries require a robust yet user-friendly system to facilitate collection of patient data. ClinCare EDC meets the industry-specific needs of these patient registries while providing built-in 21 CFR Part 11 and regulatory compliance. The feature-rich system is flexible and easy-to-use while maintaining an affordable price point.
Investigator Initiated Studies
The affordable cost structure of ClinCare EDC makes the system accessible to the investigator initiated studies of individual investigators and/or academic institutions. These studies focus on generating advances in medical and scientific knowledge through the optimization of existing therapies.
Advantages
For CROs
ClinCare EDC is designed to improve CRO efficiency, decrease internal costs, and expand their service offerings.
Advantages of ClinCare for CROs
- Flexibility
Ability to design custom features to fit study-specific goals - Fast setup
Built to facilitate rapid study build and deployment - Easy study maintenance
SaaS solution allows non technical staff to easily maintain their own EDC studies - Competitive pricing
Simple and straightforward cost structure
For Sponsors
ClinCare EDC provides sponsors with a system that is not only innovative but also practical and cost-effective.
Advantages of ClinCare for Sponsors
- Reliable
Fully validated, regulatory compliant and independently audited - Fast setup
Built to facilitate rapid study build and deployment - Affordable
Simple and straightforward cost structure - Pharmacovigilance
Timely creation of AE reports and secured web access for study pharmacovigilance - Data Transfer
Flexible and compliant to any standard data format - Customizable reports
Download data in multiple formats - Multiple studies
Ability to conduct multiple studies on the same platform simultaneously
For Participating Sites
ClinCare EDC streamlines site workflow with a user friendly design and rich feature set.
Advantages of ClinCare for Sites
- Web-based
Software as a Service (SaaS), system is available on any browser, anytime, anywhere - Modular build
Easily navigable - Web-based
Software as a Service (SaaS), system is available on any browser, anytime, anywhere - Intuitive user interface
User friendly and easy to use - High performance/ speed
Superior data quality, faster - Online queries
Full query management, both automatic and manually generated for rapid query resolution - Multi-system support
Mac, Windows, Linux, and mobile